FONT-BURGADA LAB




RESEARCH
How different oncogenic events interact during hepatocellular carcinoma (HCC) initiation.
To date, mouse models of HCC do not accurately replicate the molecular development of human liver cancer, a problem that presents an obstacle to fully understand the complex mechanisms of human HCC and poses a limit to the development of novel therapeutic approaches. We are combining mouse genetics with the use of transposons to better replicate the initiation of HCC as it develops in humans, as well as the interactions between different oncogenic mutations.
.jpg?crc=3995691234)
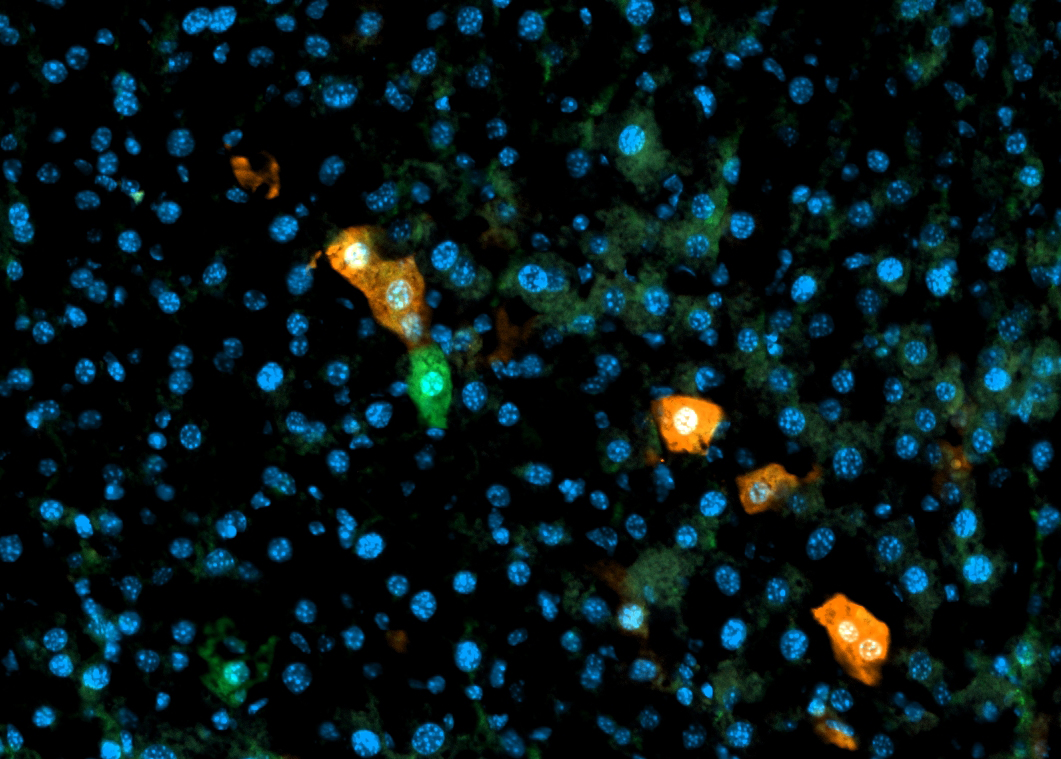
What are the molecular features of the different cholangiocarcinoma (CCA) subtypes?
CCA can be anatomically divided into three different subtypes: distal, perihilar, and intrahepatic. Distal and perihilar originate from the common bile duct, whereas intrahepatic originates from the intrahepatic bile duct cells. While these anatomical differences are understood, our lab is interested in the molecular features of each type of CCA, which have yet to be fully elucidated. We have developed an in vitro model using organoids derived from intrahepatic and common bile duct cells from mice harboring human CCA oncogenic mutations.

How can we apply liver regeneration from mouse models to human?
Our recent identification of hybrid periportal hepatocytes (HybHP) have shown an important role in the regeneration of the damaged liver in mouse. These findings form the basis of our next goal, which is to translate these results from our mouse models into human cell therapy. To achieve this, we are applying single cell sequencing to human liver in order to characterize human hybrid hepatocytes.
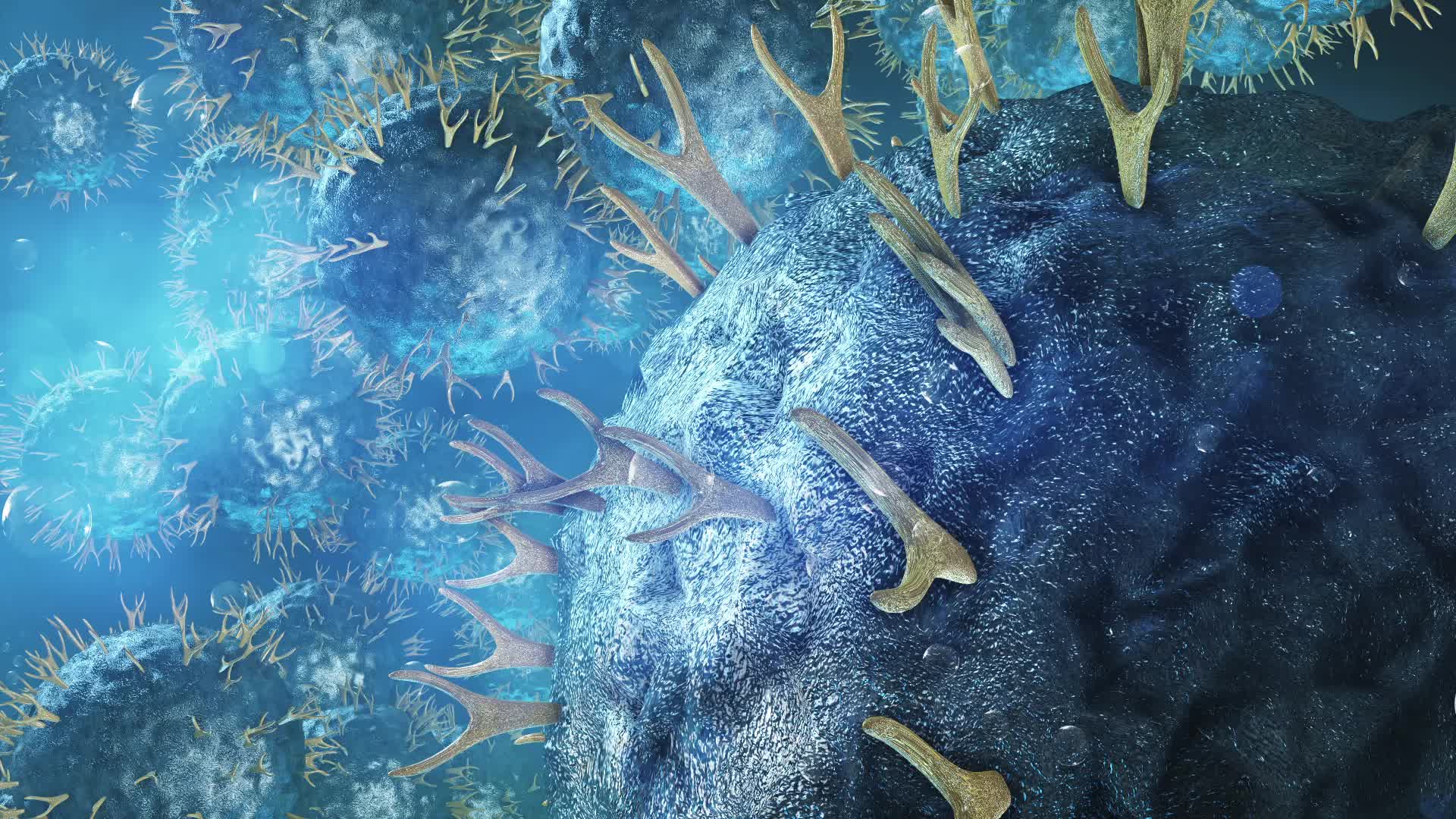
Can we inhibit metastasis by targeting cell-cell interactions?
Around 90% of the cancer related deaths result from metastatic disease rather than primary tumors. There has been a lack of interest on the specific changes that resident cells and invaded tissues undergo during cancer cell invasion. We are developing new tools to study the intimate interaction between the resident cells and the invading cancer cells to expand the current therapies on the treatment of metastasis.